US approves two gene therapies for sickle cell disease
The Hindu
The U.S. Food and Drug Administration on Friday approved a pair of gene therapies for sickle cell disease, including the first treatment based on the breakthrough CRISPR gene editing technology.
The U.S. Food and Drug Administration (FDA) on Friday approved a pair of gene therapies for sickle cell disease, including the first treatment based on the breakthrough CRISPR gene editing technology.
The agency approved Lyfgenia from bluebird bio, and a separate treatment called Casgevy by partners Vertex Pharmaceuticals and CRISPR Therapeutics.
Both the therapies were approved for people aged 12 years and older.
The Vertex/CRISPR gene therapy uses the breakthrough gene editing technology that won its inventors the Nobel Prize in 2020.
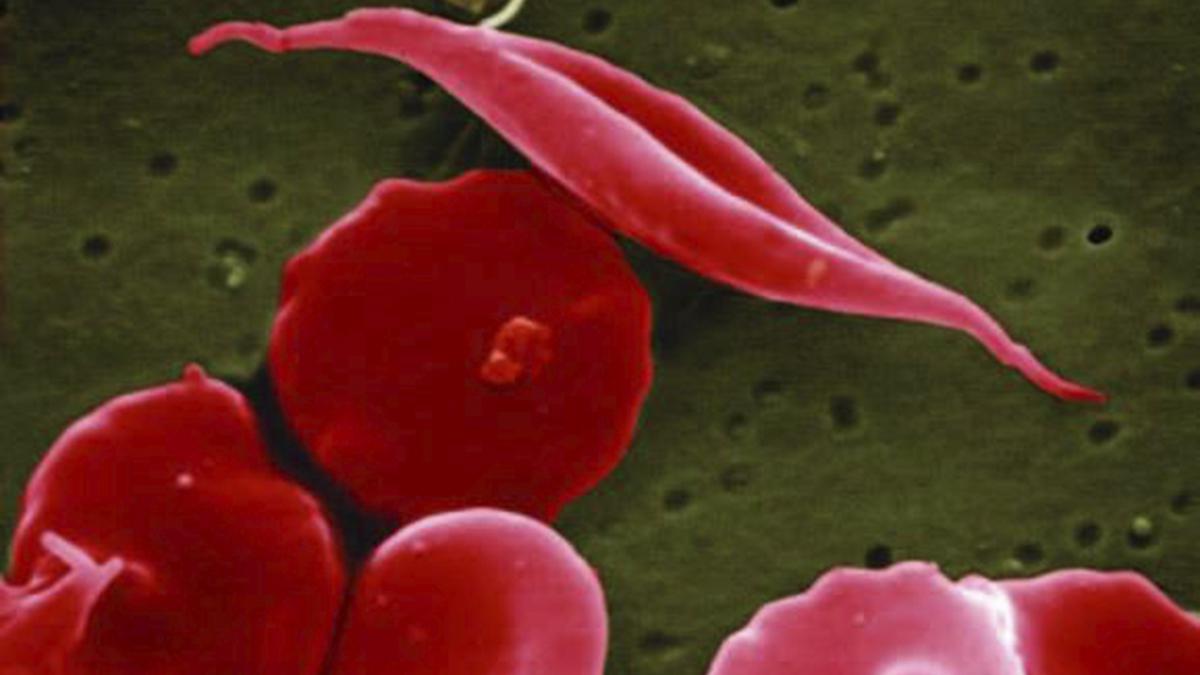
Sickle cell disease is a painful, inherited blood disorder that can be debilitating and lead to premature death. It affects an estimated 100,000 people in the United States, most of whom are Black.
In sickle cell disease, the body makes flawed, sickle-shaped hemoglobin, impairing the ability of red blood cells to properly carry oxygen to the body’s tissues.
The sickle cells tend to stick together and can block small blood vessels, causing intense pain. It also can lead to strokes and organ failure.











