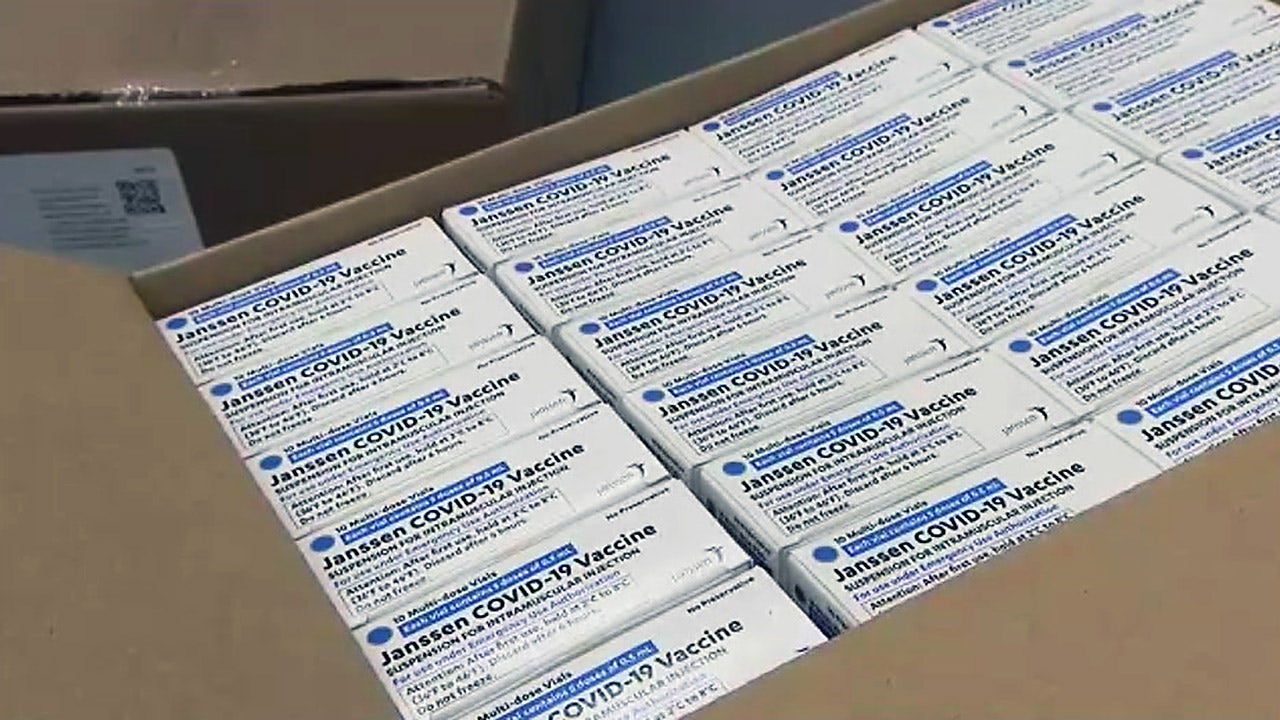
FDA recommends pause of Johnson & Johnson COVID-19 vaccine after blood clot cases
Fox News
The Food and Drug Administration together with the Centers for Disease Control and Prevention (CDC) is recommending a pause in the rollout of the Johnson & Johnson COVID-19 vaccine after several instances of a severe blood clot in recipients.
"Right now, these adverse events appear to be extremely rare," the agency said on Twitter. "Treatment of this specific type of blood clot is different from the treatment that might typically be administered. CDC will convene a meeting of the Advisory Committee on Immunization Practices (ACIP) on Wednesday to further review these cases and assess their potential significance. FDA will review that analysis as it also investigates these cases." However, until that review is completed, the FDA is "recommending this pause."More Related News













