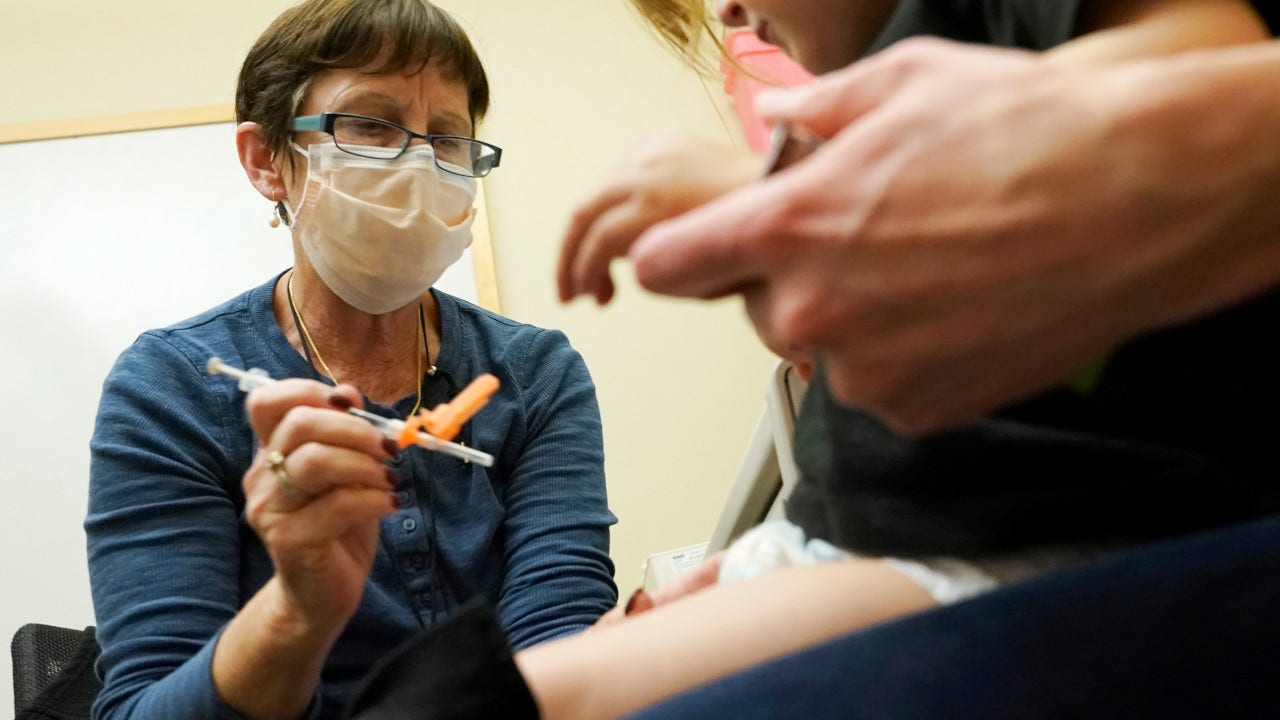
FDA authorizes bivalent COVID-19 booster shots for children under 5
Fox News
The FDA on Thursday approved the use of updated COVID-19 vaccines for children younger than 5, three days after Pfizer-BioNTech applied for an emergency use authorization.
The agency said children ages 6 months through 5 years who have received the original Moderna COVID-19 vaccine are now eligible to receive the updated booster shot which specifically targets the Omicron variant of the virus. Chris Pandolfo is a writer for Fox News Digital. Send tips to chris.pandolfo@fox.com and follow him on Twitter @ChrisCPandolfo.
Children who have not yet begun the three-dose series of the Pfizer-BioNTech vaccine or have not yet received a third dose, will now be given the bivalent booster as their third shot following two doses of the original Pfizer COVID-19 vaccine.













