U.S. FDA paves way for Pfizer COVID-19 vaccinations in young kids
CTV
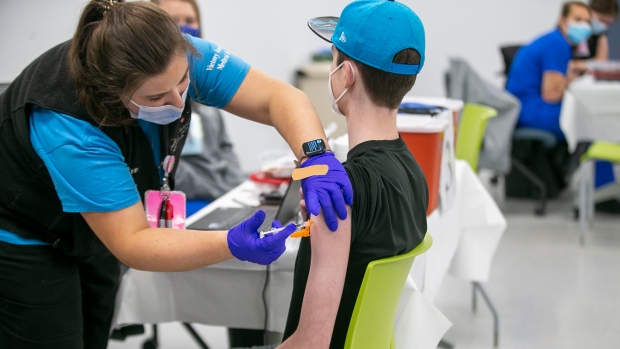
The U.S. Food and Drug Administration has paved the way for children ages 5 to 11 to get Pfizer's COVID-19 vaccine.
The FDA cleared kid-size doses -- just a third of the amount given to teens and adults -- for emergency use, and up to 28 million more American children could be eligible for vaccinations as early as next week.
One more regulatory hurdle remains: On Tuesday, advisers to the Centers for Disease Control and Prevention will make more detailed recommendations on which youngsters should get vaccinated, with a final decision by the agency's director expected shortly afterwards.
"Vaccinating younger children against COVID-19 will bring us closer to returning to a sense of normalcy," Dr. Janet Woodcock, the acting FDA commissioner, said in a statement. "Our comprehensive and rigorous evaluation of the data pertaining to the vaccine's safety and effectiveness should help assure parents and guardians that this vaccine meets our high standards."
A few countries have begun using other COVID-19 vaccines in children under 12, including China, which just began vaccinations for 3-year-olds. But many that use the vaccine made by Pfizer and its partner BioNTech are watching the U.S. decision, and European regulators just began considering the companies' kid-size doses.
