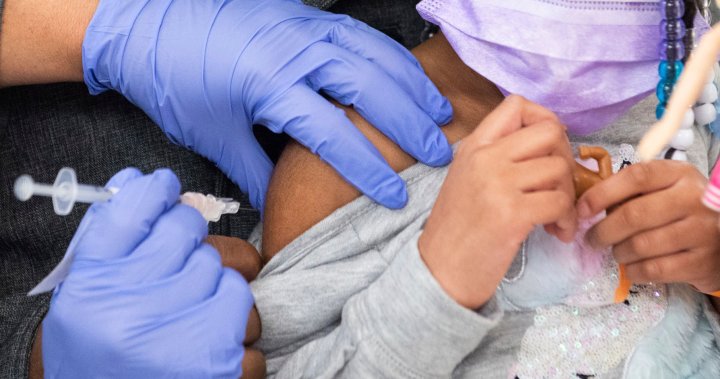
U.S. FDA advisors endorse COVID-19 vaccines for kids under 5 years old
Global News
If the FDA and CDC both give final approval to Pfizer and Moderna's shots, they could be available as soon as next week for the country's youngest children.
The first COVID-19 shots for U.S. infants, toddlers and preschoolers moved a step closer Wednesday.
The Food and Drug Administration’s vaccine advisers gave a thumbs-up to vaccines from Moderna and Pfizer for the littlest kids.
The outside experts voted unanimously that the benefits of the shots outweigh any risks for children under 5 — that’s roughly 18 million youngsters. They are the last age group in the U.S. without access to COVID-19 vaccines and many parents have been anxious to protect their little children.
If all the regulatory steps are cleared, shots should be available next week.
“This is a long-awaited vaccine,” said one panel member, Dr. Jay Portnoy of Children’s Hospital in Kansas City, Missouri. “There are so many parents who are absolutely desperate to get this vaccine and I think we owe it to them to give them a choice to have the vaccine if they want to.”
Dr. Peter Marks, FDA’s vaccine chief, opened the meeting with data showing a “quite troubling surge” in young children’s hospitalizations during the omicron wave, and noted 442 children under 4 have died during the pandemic. That’s far fewer than adult deaths, but should not be dismissed in considering the need for vaccinating the youngest kids, he said.
“Each child that’s lost essentially fractures a family,” Marks said.
While endorsing the vaccines, some panel members said they believe chances are minimal for severe illness and death in young children.












