MoreBack to News Headlines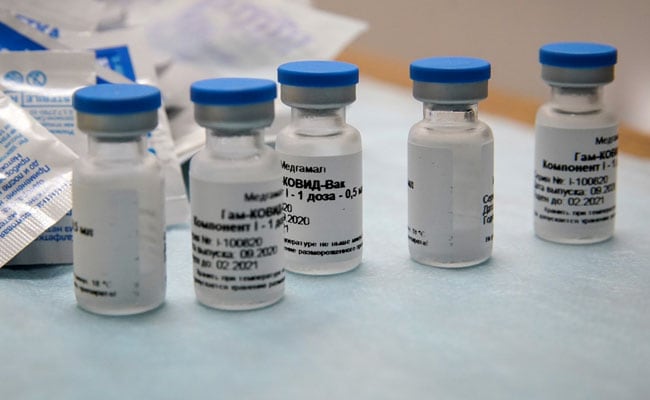

"Trial Data Clear, Transparent...": Russian Scientists Defend Sputnik V
NDTV
Concerns over phase 3 studies were raised by a group of scientists from universities in the United States, the Netherlands, Italy, France and Russia
Russian scientists leading clinical studies for the Sputnik V COVID-19 vaccine have responded to claims of "serious concerns over interim results from phase 3 trials" by stressing their data meets "clear and transparent standards... considered sufficient for regulatory review and approvals". Sputnik V, we have a problem.@sputnikvaccinehttps://t.co/3kwmKNJ7GV@TheLancetpic.twitter.com/8QdGKHSSaF Concerns over phase 3 studies - specifically data discrepancies, trial protocols, and the accuracy and quality of data from which conclusions were drawn - were raised by a group of scientists from universities in the United States, the Netherlands, Italy, France and Russia. Their note, which also flags "problematic data in the published phase 1/2 results" was published Wednesday in the 'online first' section of international medical journal The Lancet. Among the points raised was a change in primary outcome - vaccine efficacy - recorded in September. "Initially, the primary outcome was to be assessed after the first dose but the evaluation was postponed to after the second dose. The presented primary result (efficacy of 91.6%) is dependent on this change, but the reasons for the change have not been made public," the scientists wrote.More Related News

 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game