There’s still no generic Ozempic, but a lower-priced daily injected GLP-1 is coming
CNN
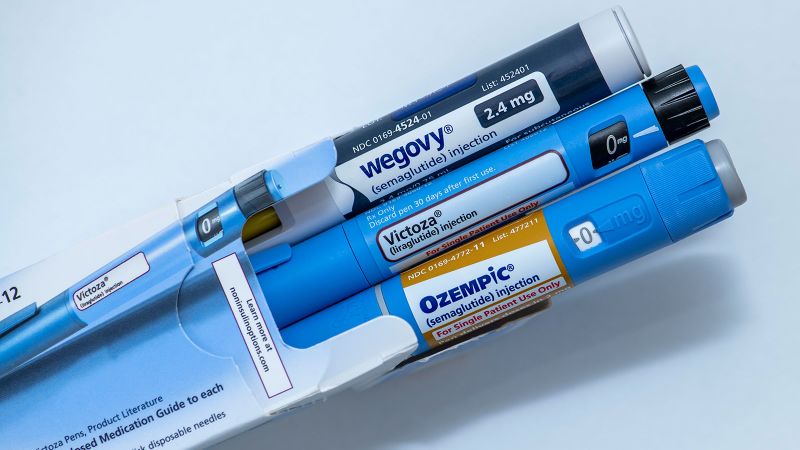
The US Food and Drug Administration on Monday approved a generic version of the daily injectable GLP-1 medicine liraglutide for people with type 2 diabetes, opening the door for lower-priced options to reach the market and help address a shortage.
The US Food and Drug Administration on Monday approved a generic version of the daily injectable GLP-1 medicine liraglutide for people with type 2 diabetes, opening the door for lower-priced options to reach the market and help address a shortage. Liraglutide, sold under the brand name Victoza for diabetes, is an earlier iteration in the same class as semaglutide, the active ingredient in Ozempic. Both are sold by Danish drug giant Novo Nordisk. The generic liraglutide is sold by Hikma Pharmaceuticals USA, and a spokesman told CNN by email that the company expects to make the drug available nationwide before the end of the year. The company didn’t disclose the planned price of the generic, noting only that it “will cost less than branded Victoza.” The branded drug costs between $500 and $815 per package, depending on the dosage, before discounts or insurance, according to Novo Nordisk, which also sells a version of liraglutide approved for obesity, called Saxenda. “There are many people on this drug, and they will benefit by another generic version of it,” Dr. Harlan Krumholz, a cardiologist at Yale University and Yale New Haven Hospital, told CNN by email. But, Krumholz noted, the newer GLP-1 drugs, which are given as weekly injections instead of daily ones, have shown stronger benefits, especially for patients with obesity – and those aren’t yet available as generics.

 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game






