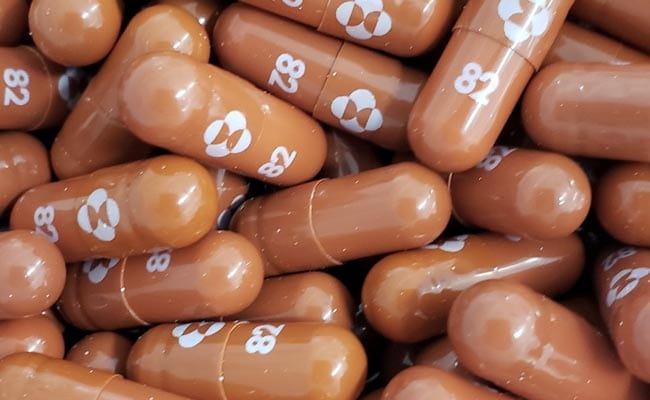
Second Covid Pill Cleared By US Today, (First Pfizer, Now Merck)
NDTV
"Today's authorization provides an additional treatment option against the COVID-19 virus in the form of a pill that can be taken orally," said FDA scientist Patrizia Cavazzoni.
The US Food and Drug Administration (FDA) on Thursday authorized Merck's Covid pill for high risk adults, a day after giving the green light to a similar pill by Pfizer.
"Today's authorization provides an additional treatment option against the COVID-19 virus in the form of a pill that can be taken orally," said FDA scientist Patrizia Cavazzoni.
The pill developed by Merck, which is known as MSD outside the US and Canada, is taken within five days of symptom onset and has been shown to reduce Covid hospitalizations and deaths by 30 percent among at-risk people.
Pfizer's pill reduced the same outcomes by 90 percent.
