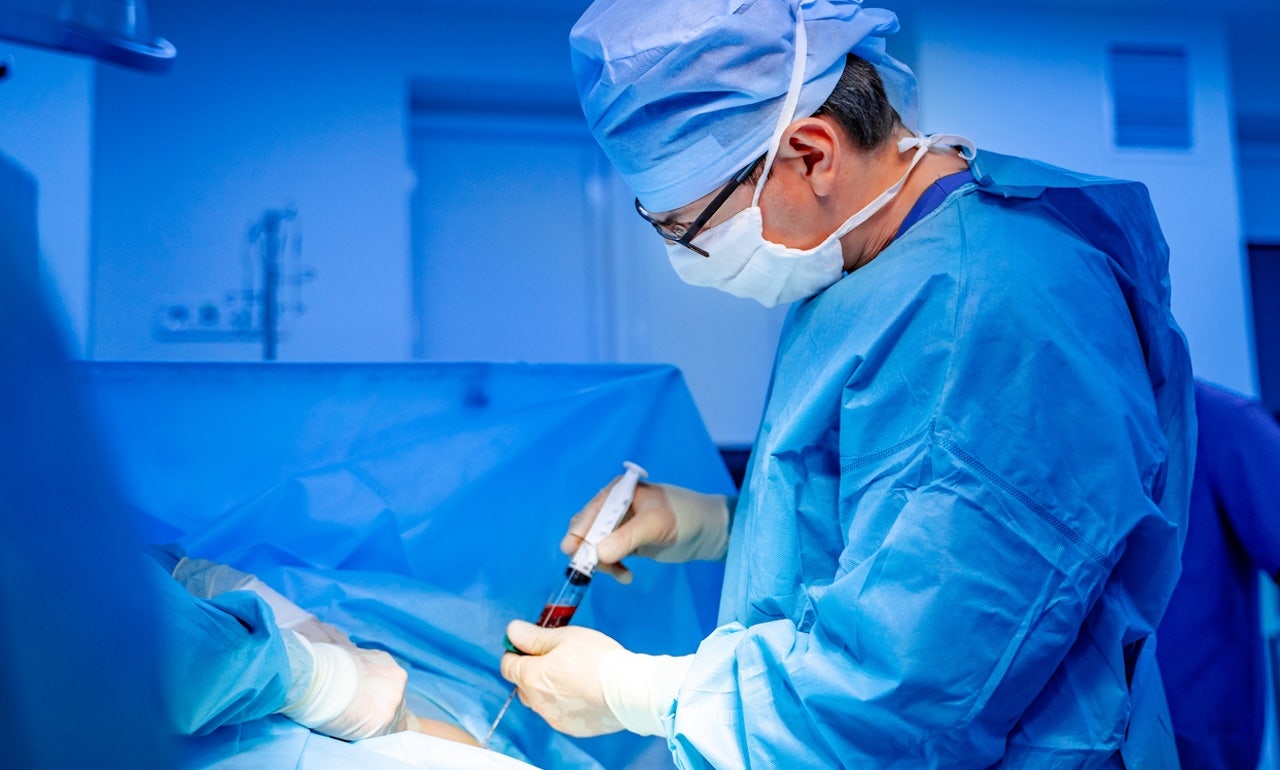
‘Remarkable’ gene-editing treatment for sickle cell disease is approved by FDA
Fox News
The FDA has approved the world’s first gene-edited treatment for symptoms of sickle cell disease. Experts weigh in on the potential benefits and limitations.
"CASGEVY’s approval by the FDA is momentous," Reshma Kewalramani, M.D., chief executive officer and president of Vertex Pharmaceuticals in Boston, said in a statement provided to Fox News Digital. "CASGEVY is a first-in-class treatment that offers the potential of a one-time transformative therapy for eligible patients with sickle cell disease."
"It is the first CRISPR-based gene-editing therapy to be approved in the U.S."
More Related News

 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game
 Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game
 Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game