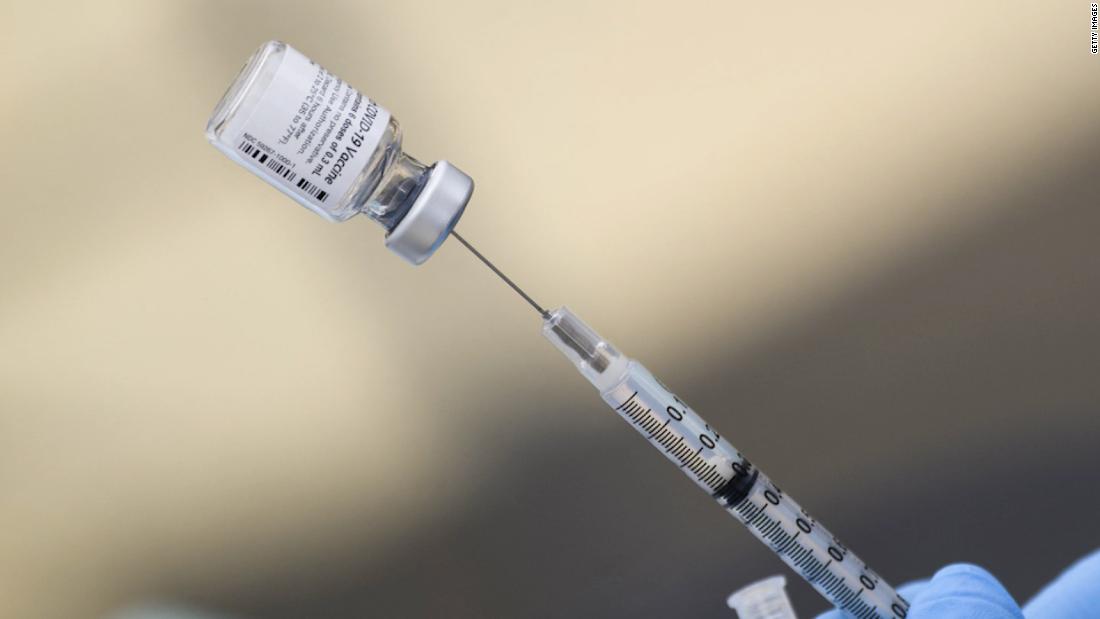
Pfizer/BioNTech submit initial data on Covid-19 vaccine for people ages 5 to 11 to FDA, but aren't seeking EUA yet
CNN
Pfizer and BioNTech said Tuesday they have submitted Covid-19 vaccine data on children ages 5 to 11 to the US Food and Drug Administration for initial review, but are not yet seeking emergency use authorization.
A formal submission to request EUA for the vaccine is expected to follow in the coming weeks, the companies said in a statement.
Submissions to the European Medicines Agency and other regulatory authorities are also planned, they said.

Immigration and Customs Enforcement officials will be given access to the personal data of the nation’s 79 million Medicaid enrollees, including home addresses and ethnicities, to track down immigrants who may not be living legally in the United States, according to an agreement obtained by The Associated Press.





















 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game










