Pfizer asks FDA to allow COVID shots for children ages 5 to 11
CBSN
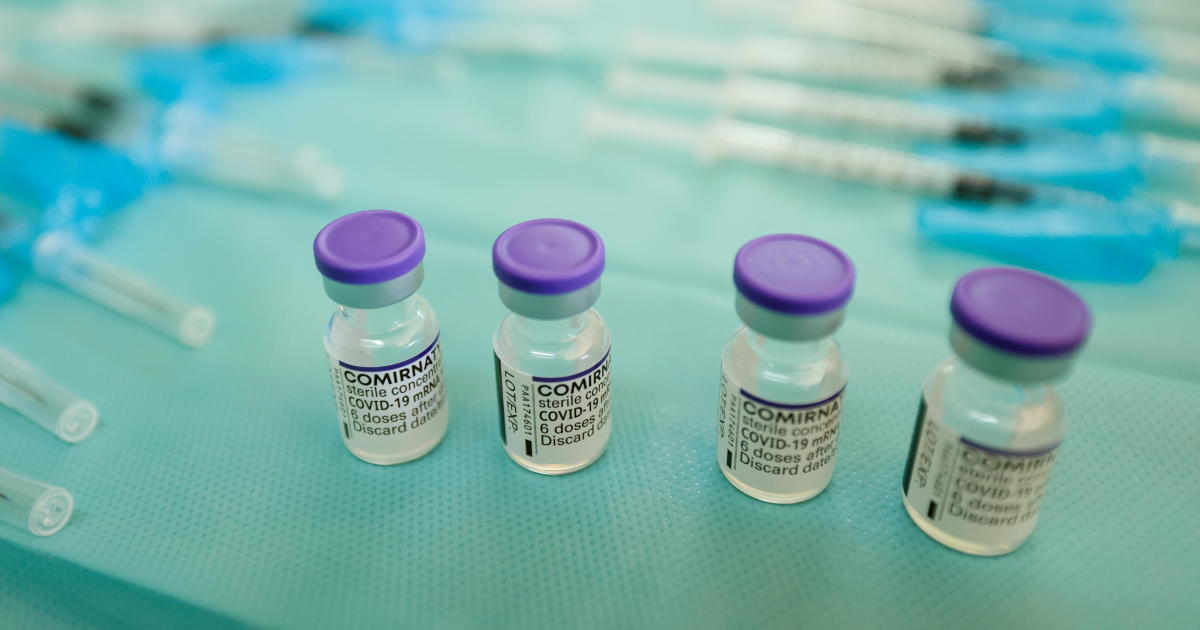
Pfizer asked the U.S. government Thursday to allow use of its COVID-19 vaccine in children ages 5 to 11 -- and if regulators agree, shots could begin within a matter of weeks.
Many parents and pediatricians are clamoring for protection for children younger than 12, today's age cutoff for the vaccine made by Pfizer and its German partner BioNTech. Not only can youngsters sometimes get seriously ill, but keeping them in school can be a challenge with the coronavirus still raging in poorly vaccinated communities.
Pfizer announced in a tweet that it had formally filed its application with the Food and Drug Administration.

Washington — Internal friction with the Justice Department team that fights monopolies has led to private conversations in the Trump administration about whether to push out some staff in the antitrust division or to work to smooth out the issues, according to multiple sources familiar with the situation.





















 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game










