Ozempic, Wegovy not associated with higher risk of suicidal ideation in large review of US health records
CNN
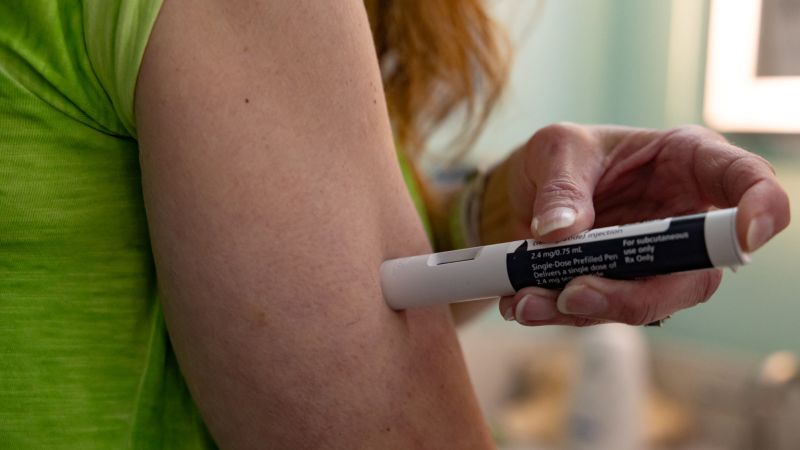
Use of the medicine semaglutide for weight loss or type 2 diabetes wasn’t associated with a higher risk of suicidal ideation than other medicines for those conditions in a large new review of US health records that was backed by the National Institutes of Health.
Use of the medicine semaglutide for weight loss or type 2 diabetes wasn’t associated with a higher risk of suicidal ideation than other medicines for those conditions in a large new review of US health records that was backed by the National Institutes of Health. The researchers used a database that includes more than 100 million patient records to assess the risks of suicidal ideation among people using the drug, sold as Ozempic for type 2 diabetes and Wegovy for weight loss. The results were published Friday in the journal Nature Medicine. Dr. Rong Xu, an author of the study and professor of biomedical informatics at Case Western Reserve University School of Medicine, said she decided to look into the issue after European regulators opened a probe into semaglutide and reports of suicidal thoughts last summer. The US Food and Drug Administration said this week that it’s also conducting its own investigation. Xu and her fellow researchers, including National Institute on Drug Abuse Director Dr.Nora Volkow, compared cases of suicidal ideation among people taking semaglutide with those taking other medicines for weight loss or diabetes. “We observed a lower incidence of suicidal ideations in patients who had taken semaglutide than in patients who were treated with non-GLP1R-targeting medications for the same conditions,” Volkow wrote in an email, referring to the way semaglutide works to mimic a hormone called GLP-1. The analysis included more than 240,000 people with obesity and more than 1.5 million with type 2 diabetes. It looked at the risk of suicidal ideation within six months of starting the medicines, as well as at longer time points.





















 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game