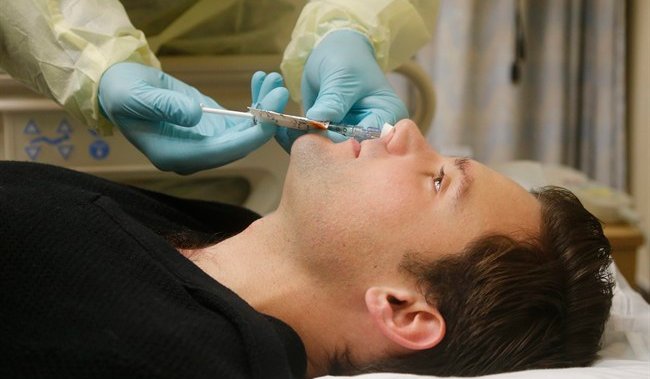
Nasal vaccine to treat Alzheimer’s disease to be tested in U.S. clinical trial
Global News
If successful, the vaccine could potentially be given to people years from now who are at risk for the disease.
A first-of-its-kind clinical trial is underway in the United States, examining the effectiveness of a nasal vaccine for the treatment of Alzheimer’s disease.
The study, to be conducted at Brigham and Women’s Hospital in Boston, began recruiting participants this week, and is the first human trial of a nasal vaccine for the disease.
“If clinical trials in humans show that the vaccine is safe and effective, this could represent a nontoxic treatment for people with Alzheimer’s, and it could also be given early to help prevent Alzheimer’s in people at risk,” Dr. Howard Weiner, co-director of the Ann Romney Center for Neurologic Diseases at the Brigham, who will lead the research, said in a statement.
The vaccine will test an immunotherapy drug called Protollin, which works by stimulating the body’s immune system, targeting a build-up of beta amyloid protein plaques in the brain. Scientists believe these build-ups are one of the ways human brain cells are prevented from working properly in Alzheimer’s.
According to a hospital press release, the phase one trial will involve 16 participants between the ages of 60 and 85 who have early symptomatic Alzheimer’s disease but are otherwise in good health. They will each receive two doses of Protollin, one week apart.
Protollin has been found to be safe in other vaccines, and Weiner told the Boston Herald that his pre-trial research showed that a nasally administered dose provides the best results, and did not reveal any major side effects.
Weiner told the Herald the vaccine “can help with the disease and even more importantly, it can be given to people who are at risk for the disease or have it and don’t know it.”
He said many people in their 50s and 60s show signs of developing Alzheimer’s in brain imaging, despite having normal cognition.













