Moderna completes submission for full FDA approval of Covid-19 vaccine; Pfizer seeks approval for booster dose
CNN
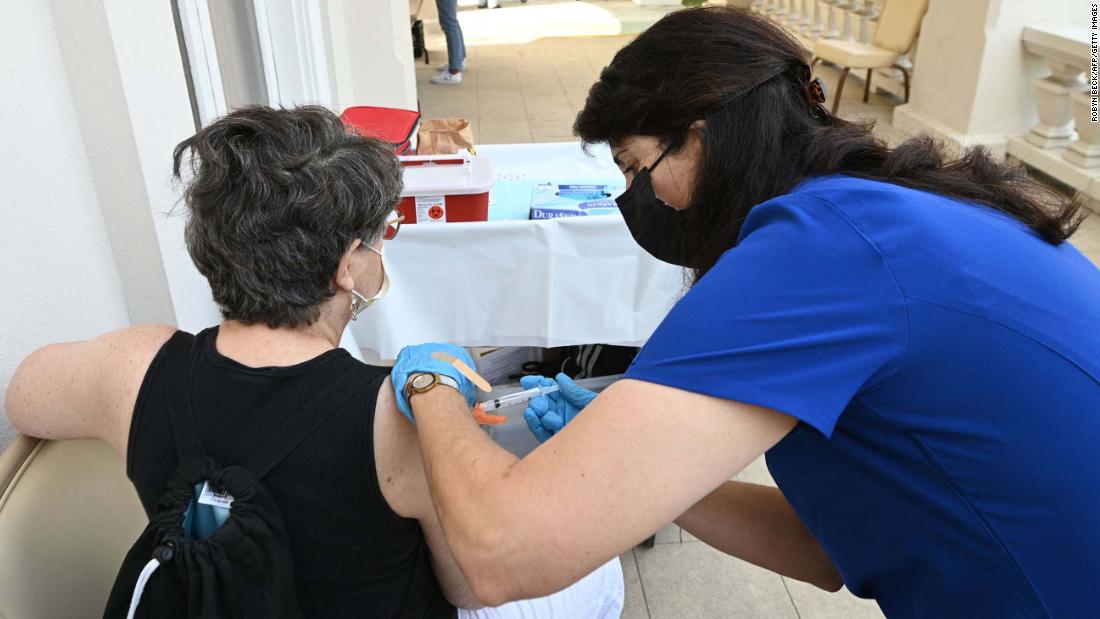
Moderna announced on Wednesday it has completed its submission to the US Food and Drug Administration for full approval of its Covid-19 vaccine for people age 18 and older, and Pfizer and BioNTech announced they have begun submitting data for full FDA approval of a third dose of their vaccine.
Moderna said it has requested priority review from the FDA. The company began submitting data for its Biologics License Application, or BLA, to the FDA in June.
The two men killed as they floated holding onto their capsized boat in a secondary strike against a suspected drug vessel in early September did not appear to have radio or other communications devices, the top military official overseeing the strike told lawmakers on Thursday, according to two sources with direct knowledge of his congressional briefings.

Defense Secretary Pete Hegseth risked compromising sensitive military information that could have endangered US troops through his use of Signal to discuss attack plans, a Pentagon watchdog said in an unclassified report released Thursday. It also details how Hegseth declined to cooperate with the probe.





















 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game









