Moderna asks FDA to evaluate its COVID-19 vaccine for booster shot
CBSN
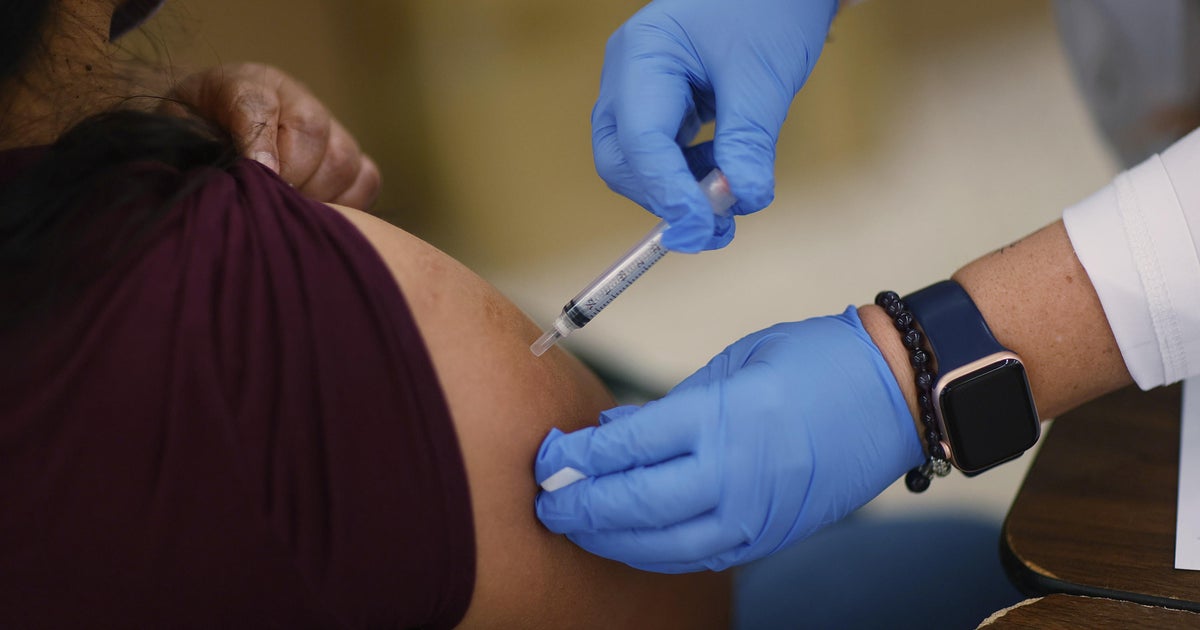
Moderna said on Wednesday it is asking the Food and Drug Administration to evaluate its COVID-19 vaccine for use in a booster shot. The drugmaker's own testing found that a third shot created "robust antibody responses" to the Delta variant, which is spreading across the U.S.
Moderna also plans to submit data to the European Medicines Agency and other regulatory authorities around the world in the next few days. The biotech company's submission the FDA comes as the Biden administration recommends that most Americans get a booster shot eight months after their second dose of a COVID-19 vaccine. Once the vaccines are fully approved by the FDA and signed off by the Centers for Disease Control and Prevention, the boosters could be administered.More Related News
