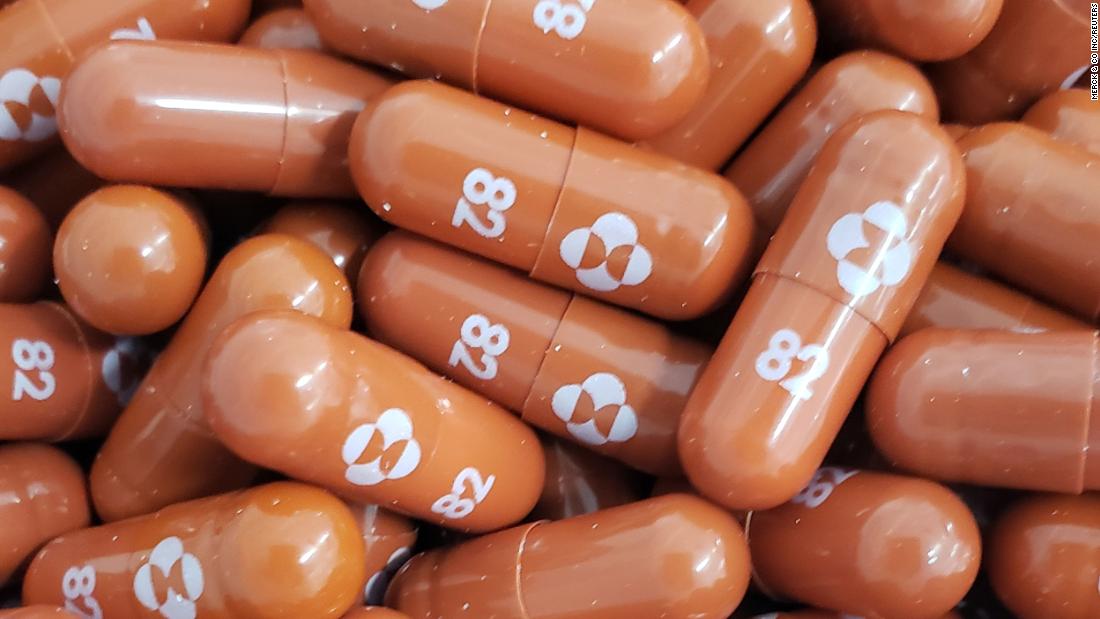
Merck seeks FDA emergency use authorization for antiviral Covid-19 treatment molnupiravir
CNN
Merck said it is seeking FDA emergency use authorization for its experimental antiviral Covid-19 treatment, molnupiravir.
If authorization is granted, the drug, made by Merck and Ridgeback Biotherapeutics, would be the first oral antiviral treatment to fight Covid-19. It comes in capsule form.
Merck said it is asking for authorization for the capsules to treat infected adults who are at risk of progressing to severe Covid-19 disease or hospitalization. Its submission is based on a study that was stopped at the interim point because the drug was working so well in more than 700 patients randomly assigned to take either molnupiravir or a placebo.

Texas judge orders Attorney General Ken Paxton’s divorce records unsealed amid heated Senate primary
Court documents detailing the divorce of Republican U.S. Senate candidate and Texas Attorney General Ken Paxton and his wife, state Sen. Angela Paxton, were released Friday by order of a judge, months after she filed citing “biblical grounds.”












