Kala Pharmaceuticals shares soar after FDA approves trial for drug to treat PCED
CBSN
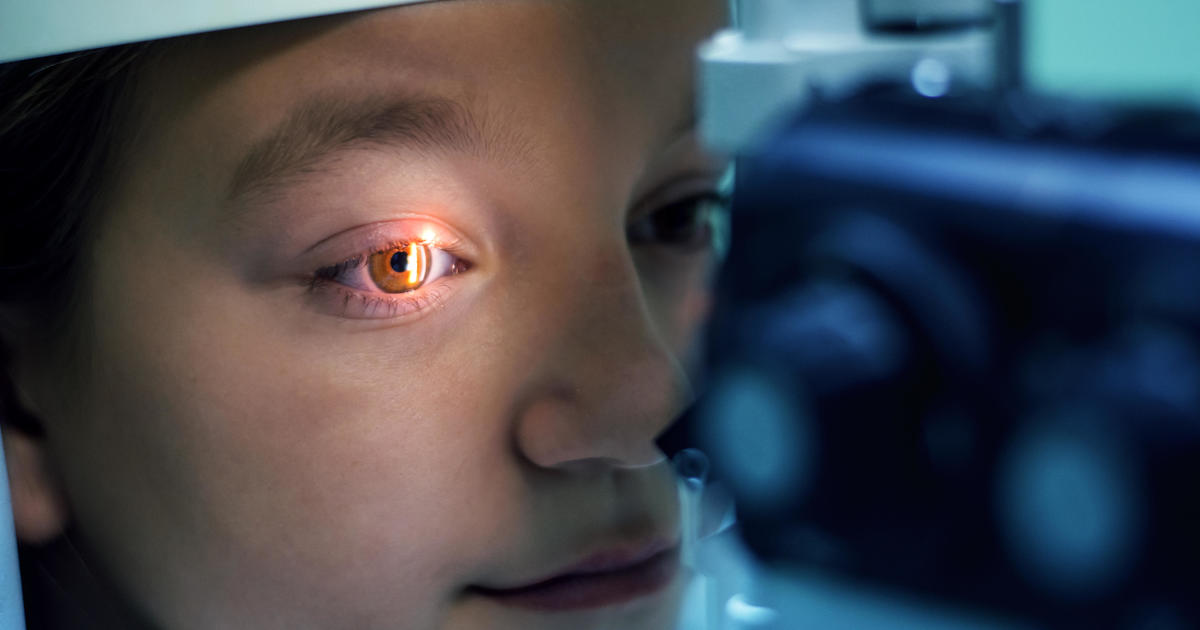
Shares of Kala Pharmaceuticals surged Wednesday after the small biotechnology company announced a drug candidate for a rare eye disease.
The Food and Drug Administration has accepted Kala's investigational drug application for KPI-012, a potential treatment for persistent corneal epithelial defect, or PCED, the Arlington, Massachusetts-based company announced on Tuesday.
Kala shares soared $9.45, or 266%, Wednesday to $13.52.
More Related News


