Johnson & Johnson HIV vaccine trial fails mid-stage study
ABC News
A Johnson & Johnson HIV vaccine candidate failed to reduce the risk of infection in a clinical trial among women in southern Africa.
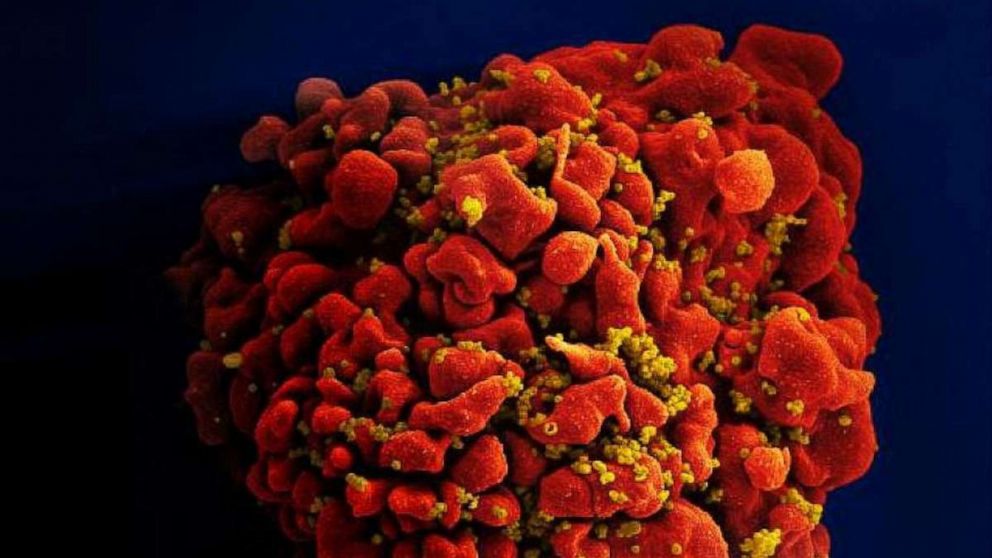
In yet another setback in the decadeslong scientific quest for an HIV vaccine, a Johnson & Johnson HIV vaccine candidate failed to reduce the risk of infection in a clinical trial among women in southern Africa. The would-be vaccine uses the same underlying technology used successfully for COVID-19 and Ebola viruses, but this recent high-profile failure is another example of immense challenge of creating a vaccine against HIV. The trial, called Imbokodo, was co-sponsored by the Bill & Melinda Gates Foundation and the U.S. National Institutes of Health. It included more than 2,600 women living in five African countries where women and girls have a high risk of HIV infection. The vaccine was safe, researchers said, but ultimately, efficacy was only 25% -- meaning people who received the vaccine had a slightly smaller risk of developing HIV, but the difference was so slight that the result might be chalked up to random chance. Prominent scientists, including Dr. Anthony Fauci, have been searching for an effective HIV vaccine since the virus, which attacks the immune system and leads to a disease called AIDS if left untreated, was first identified in the 1980s. Today, nearly 38 million people are living with HIV worldwide. Although effective treatments can now help people infected with HIV live long and healthy lives, there is still no vaccine that can prevent infection.More Related News
