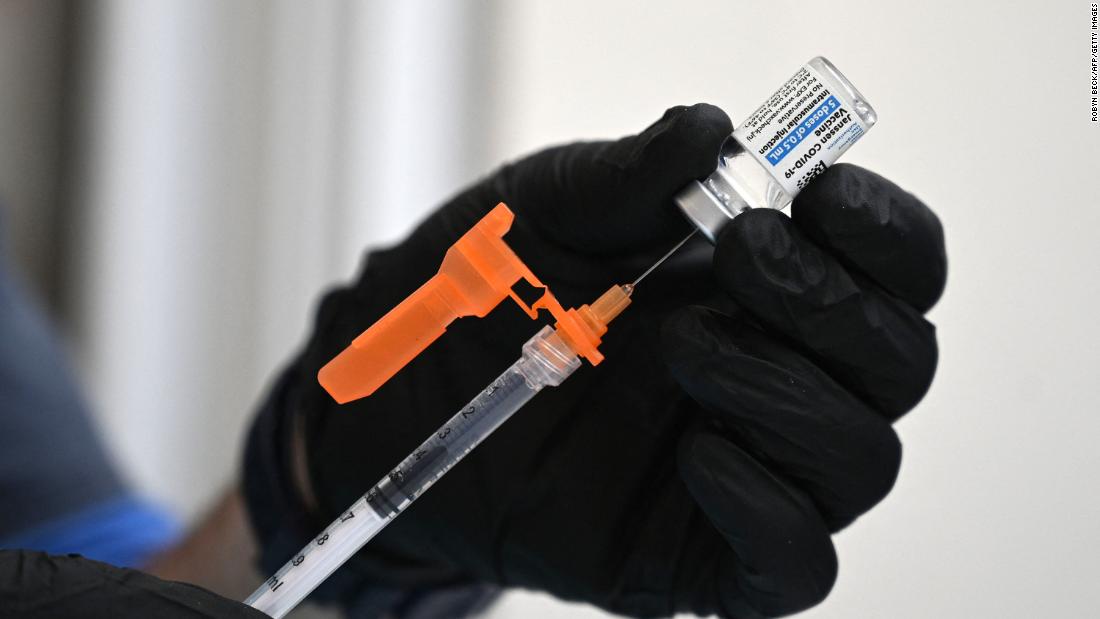
Johnson & Johnson asks FDA to authorize Covid-19 vaccine booster shots
CNN
Johnson & Johnson has asked the FDA to authorize booster shots for its coronavirus vaccine, but has left it up to the FDA and the US Centers for Disease Control and Prevention to decide just who should get their boosters and when.
"We're describing the data to them," Dr. Mathai Mammen, head of global research and development for J&J's vaccine arm, Janssen, told CNN.
"The process is not that we asked for a very specific interval -- we're providing them data and we're going to be presenting to the committee. They'll take all that into consideration when they ultimately decide on an appropriate interval."

More photos from Epstein’s estate released by House Democrats as deadline to release DOJ files looms
Democrats on the House Oversight Committee released photos from Jeffrey Epstein’s estate Thursday — the latest in a series of intermittent disclosures that have fueled significant political intrigue in recent weeks about who may have been associated with the convicted sex offender.












