‘It’s a game-changer’: New drug to protect babies from RSV approved
Global News
Health Canada has approved a new antibody drug to help protect babies from serious illness caused by respiratory syncytial virus, or RSV.
Health Canada has approved a new antibody drug to help protect babies from serious illness caused by respiratory syncytial virus, or RSV.
Nirsevimab, also known by its brand name Beyfortus, was authorized on April 19. It was developed by AstraZeneca and Sanofi.
Nirsevimab is “a monoclonal antibody to prevent serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) infection in newborns and infants during their first RSV season,” Health Canada spokesman Mark Johnson said in an email to The Canadian Press on Friday.
The drug, which is given by injection, is also authorized for children up to two years of age if they are at risk of serious infection, he said.
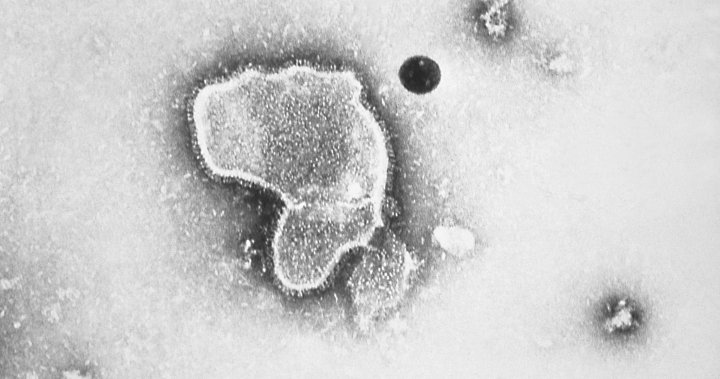
Monoclonal antibodies are made in a laboratory to mimic natural antibodies to prevent or treat diseases.
Nirsevimab attaches to a protein on the surface of the virus and hinders its ability to enter the body’s cells, especially those in the lungs, according to the European Medicines Agency, a regulatory body that last fall approved the drug for use in the European Union.
Canada already offers the monoclonal antibody palivizumab — also known by the brand name Synagis — to premature babies because they are more vulnerable to serious illness from RSV. The National Advisory Committee on Immunization (NACI) does not recommend palivizumab for healthy babies.
Palivizumab has to be injected about once a month — up to four times — during RSV season to remain effective. Nirsevimab requires only one dose that lasts the entire RSV season.


















