ICMR seeks to provide oral formulation of hydroxyurea to treat sickle cell disease in children
The Hindu
The Indian Council of Medical Research (ICMR) has invited Expressions of Interest (EoI) from eligible organisations for the “joint development and commercialisation” of low dose or paediatric oral formulation of hydroxyurea to treat sickle cell disease in India.
The Indian Council of Medical Research (ICMR) has invited Expressions of Interest (EoI) from eligible organisations for the “joint development and commercialisation” of low dose or paediatric oral formulation of hydroxyurea to treat sickle cell disease in India.
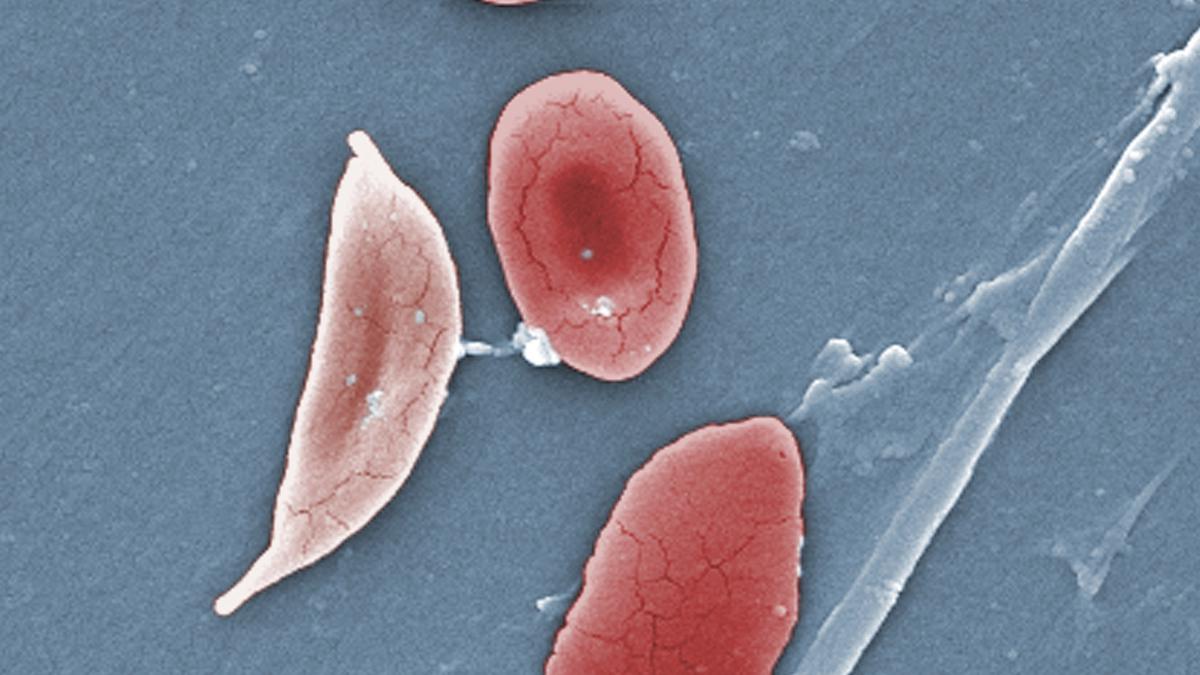
India has the highest prevalence of sickle cell disease in South Asia, and over 20 million sickle cell affected individuals reside in the country. While most pharmaceutical companies in India market 500 mg capsules or 200 mg tablets of hydroxyurea, the biggest challenge in treatment is that it’s not available in the suspension form for effective use in the case of paediatric patients, the ICMR said.
Also Read:How subpar treatment options allow sickle cell disease to persist | Explained
Sickle cell disease is one of the most common monogenic disorders of haemoglobin, and hydroxyurea, a myelosuppressive agent, is an effective drug for treating patients of sickle cell disease, and thalassemia. The ICMR said that since only high dosage hydroxyurea tablets are available, initiating a low dose treatment becomes a tedious task for service providers, as the capsule or tablet has to be broken down appropriately to be administered in accordance with body weight, thereby risking the efficacy available with measured doses.
“Thus, there is a need for paediatric formulation of HU (hydroxyurea), considering the number of SCD (sickle cell disease) cases in India and in view of the launch of the National Mission to eliminate Sickle Cell Anaemia/SCD (by 2047),’’ the council said.
The ICMR, which is the apex biomedical research body in the country, also said that in India, according to the National Health Mission’s guidelines, healthcare providers initiate hydroxyurea therapy to only symptomatic sickle cell disease patients among children both because of the lack of availability of paediatric doses as well as the fear of toxicity.
Also Read: Indigenous drug for sickle cell disease developed











