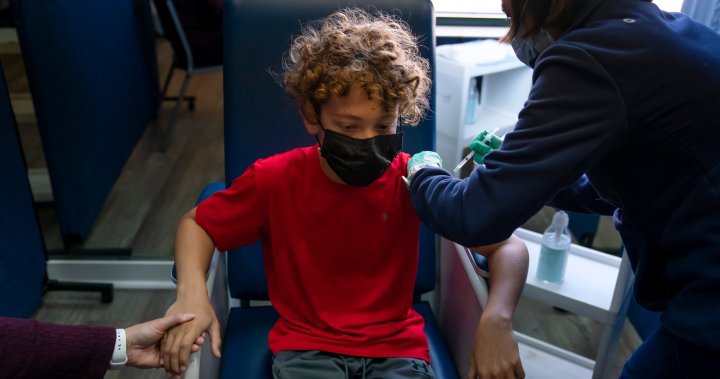
Health Canada to approve Pfizer COVID-19 vaccine for kids 5-11 on Friday: sources
Global News
Some Canadian parents desperate to get their children vaccinated are taking them to the U.S., where the Pfizer vaccine was approved for use in kids aged five to 11 on Nov. 2.
Health Canada is expected to announce Friday that it has approved the Pfizer COVID-19 vaccine for use in Canadian children between the ages of five and 11, a key demographic as the fight to control the spread of the virus ramps up ahead of winter.
Global News has confirmed that officials at the health agency are poised to announce their approval, which comes as COVID-19 cases continue fluctuating across much of the country amid a widespread shift to indoors caused by the colder weather.
Children aged five to 11 have been unable to get vaccinated so far.
That’s caused significant challenges for families as schools attempt to crack down on symptoms like coughs to mitigate the spread of the highly contagious Delta variant.
The Toronto Star first reported on the expected approval on Thursday.
READ MORE: Desperate for COVID-19 vaccines, some Canadian parents taking their kids to U.S.
Some Canadian parents desperate to get their children vaccinated have resorted to taking them south of the border, where the Pfizer vaccine was approved for use in kids aged five to 11 on Nov. 2.
Rochelle Walensky, director of the U.S. Centers for Disease Control and Prevention, called the move an “important step” in that country’s fight against COVID-19.











