Health Canada OKs AstraZeneca’s Evusheld drug for COVID-19 prevention
Global News
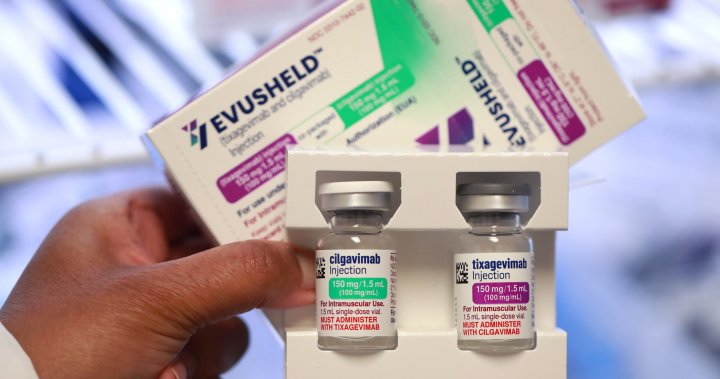
AstraZeneca's Evusheld contains lab-made antibodies designed to linger in the body for months to contain the virus in case of an infection.
Canada on Thursday authorized British drugmaker AstraZeneca’s antibody-based therapy for preventing COVID-19 infections, giving itself another weapon against the disease as cases rise in the country.
Health Canada has cleared the drug – Evusheld – for use in individuals aged 12 years and older who are immune compromised and unlikely to mount an adequate immune response to COVID-19 vaccination or for whom COVID-19 vaccination is not recommended.
While vaccines rely on an intact immune system to develop targeted antibodies and infection-fighting cells, Evusheld contains lab-made antibodies designed to linger in the body for months to contain the virus in case of an infection.
The therapy has already been authorized in the United States and its use has also been recommended by the European Medicines Agency.











