MoreBack to News Headlines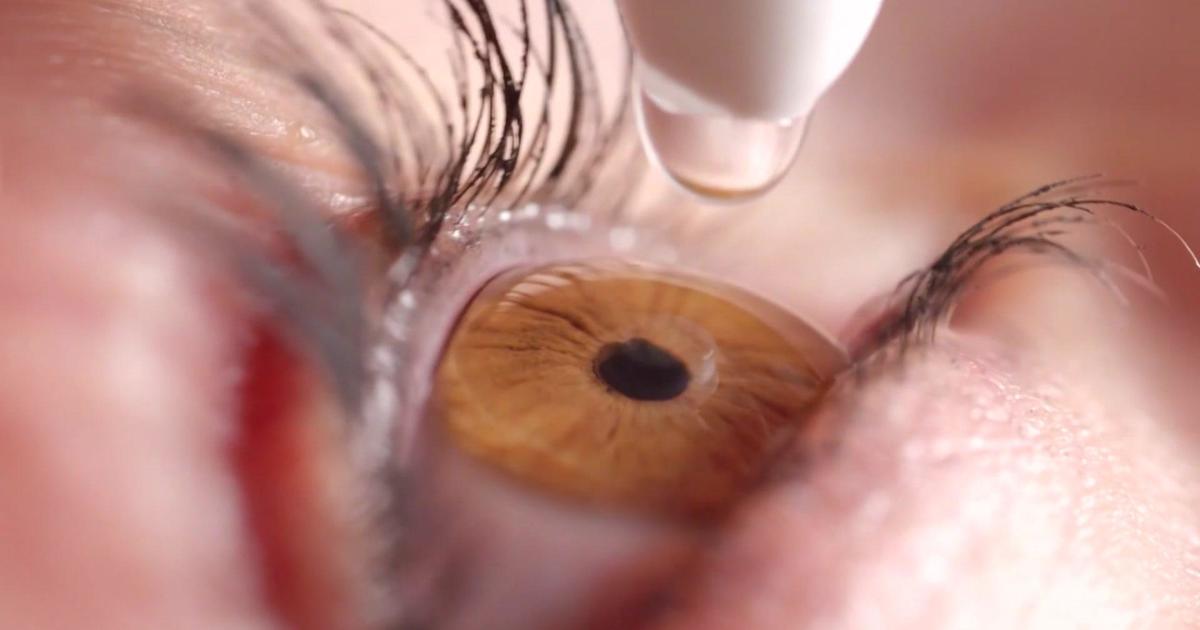

FDA warns of contaminated copycat eye drops
CBSN
The Food and Drug Administration is warning the public against copycat eye drops because of the risk of infection.
South Moon, Rebright and FivFivGo eye drops are offered in packaging that could easily be mistaken for Bausch + Lomb's Limify brand eye drops, an over-the-counter product approved for redness relief, the FDA said on Wednesday.
The copycat products claim to address conditions like glaucoma, which is treated with prescription drugs or surgery, the agency noted.
More Related News

 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game