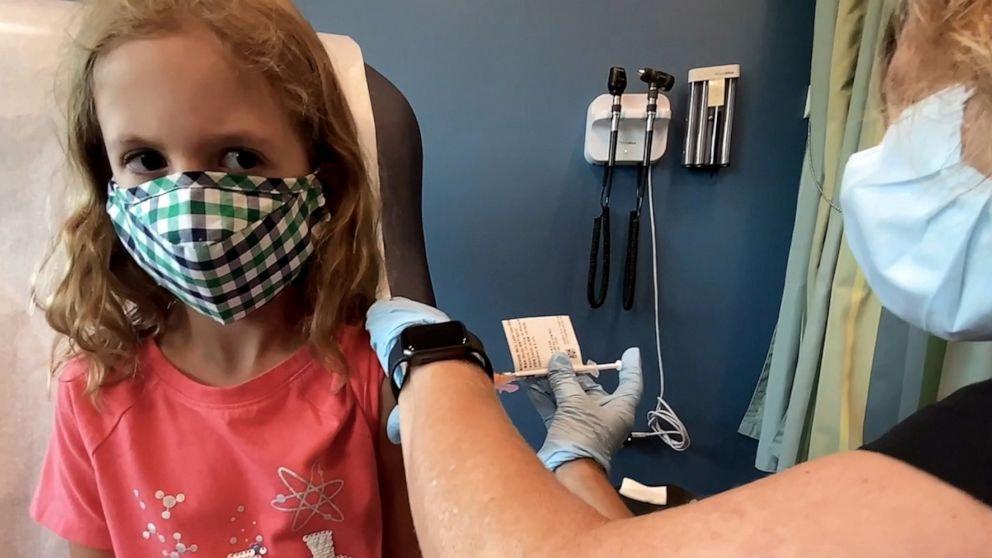
FDA panel meets to discuss vaccines for kids, kicking off authorization process
ABC News
An advisory panel at the Food and Drug Administration will vote on Tuesday whether to move forward with authorizing vaccines for children ages 5-11.
An advisory panel at the Food and Drug Administration will vote Tuesday on whether to move forward with authorizing vaccines for children ages 5-11.
The vote will be the first step in a regulatory process for the two-shot Pfizer vaccine for kids. If the panel votes in favor of the vaccine after reviewing Pfizer's data from clinical trials, the process will move to the Centers for Disease Control and Prevention.
If both agencies support the data, kids could be able to get their first shots in early November.
"If all goes well, and we get the regulatory approval, and the recommendations from the CDC, it's entirely possible, if not, very likely, that vaccines will be available for children from 5 to 11 within the first week or two of November," Dr. Anthony Fauci, chief medical adviser for the White House, said in an interview on Sunday on ABC's "This Week."
