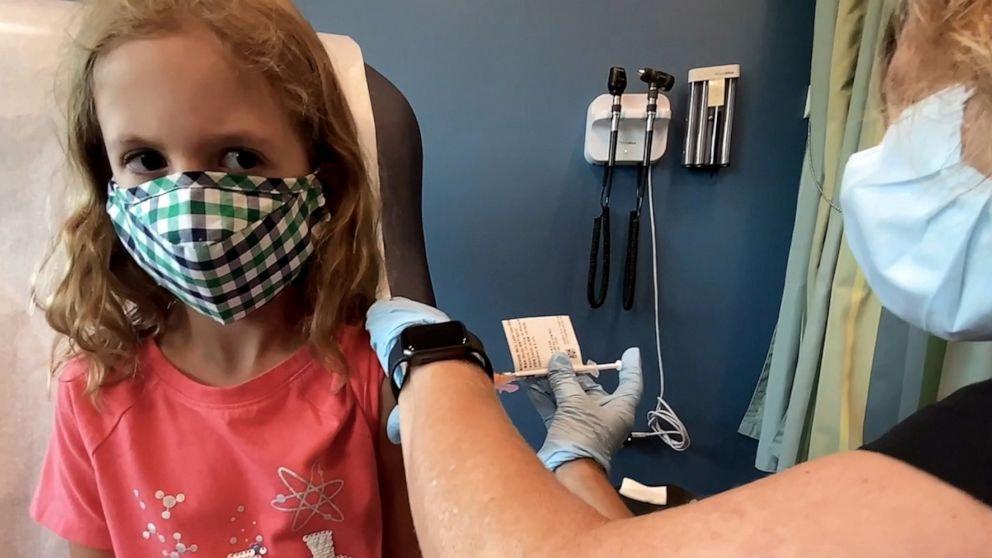
FDA panel greenlights vaccines for kids, paving the way for authorization
ABC News
An advisory panel at the Food and Drug Administration voted on Tuesday to move forward with the vaccine authorization process for children ages 5-11.
Vaccines for 28 million American children are on the way to authorization after an advisory panel at the Food and Drug Administration voted in support of the Pfizer vaccine for kids 5-11 on Tuesday afternoon.
The vote was the first step in a regulatory process for the two-shot Pfizer vaccine that could allow kids to get their first shots in early November and become fully immunized by early December.
Next, leaders of the FDA have the chance to officially sign off, potentially as soon as Tuesday night. If and when this happens, the White House will begin shipping doses, senior officials told governors on a call Tuesday afternoon that was obtained by ABC News.
But there are still more steps before shots go into arms: If authorized by the FDA, the process would move to the Centers for Disease Control and Prevention next Tuesday, when a CDC panel meets to discuss the same data reviewed by the FDA advisers.
