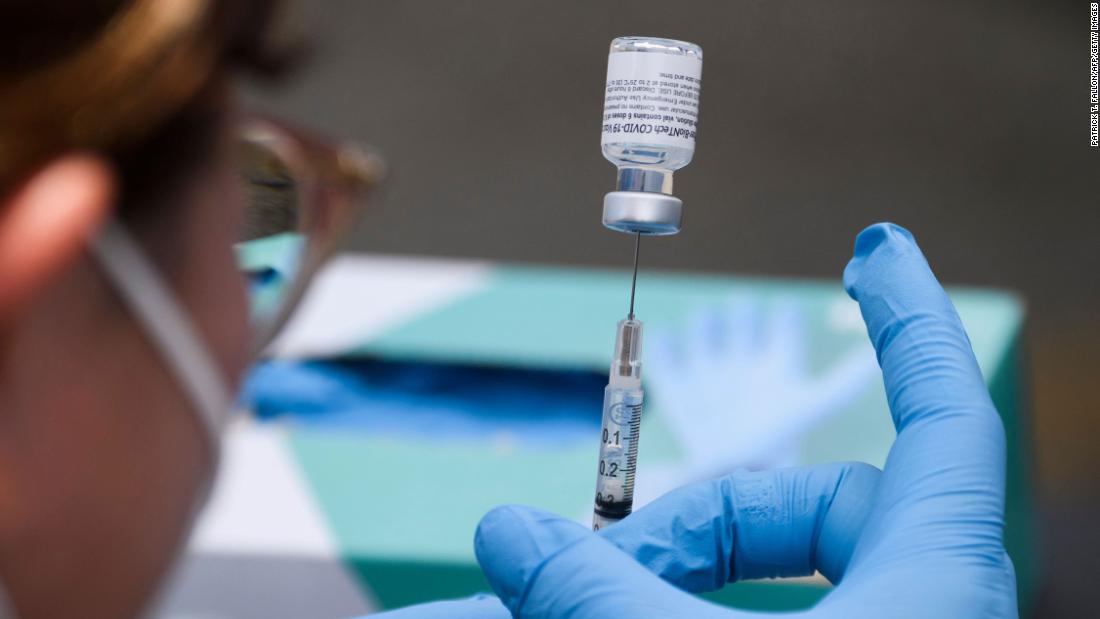
FDA expected to authorize Covid-19 vaccine booster shots for immunocompromised people within the next 48 hours
CNN
The US Food and Drug Administration is expected to announce within the next 48 hours that it is authorizing Covid-19 vaccine booster shots for people who are immunocompromised, according to a source familiar with the discussions.
This would be a third shot of the current two-dose Pfizer and Moderna vaccines. That announcement could slide, the source cautioned, but this is the current timing. "The FDA is closely monitoring data as it becomes available from studies administering an additional dose of the authorized COVID-19 vaccines to immunocompromised individuals," an FDA spokesperson told CNN. "The agency, along with the CDC, is evaluating potential options on this issue, and will share information in the near future."More Related News

A little-known civil rights office in the Department of Education that helps resolve complaints from students across the country about discrimination and accommodating disabilities has been gutted by the Trump administration and is now facing a ballooning backlog, a workforce that’s in flux and an unclear mandate.












