FDA chief calls for probe of talks between agency and Biogen before Alzheimer's drug was approved
CBSN
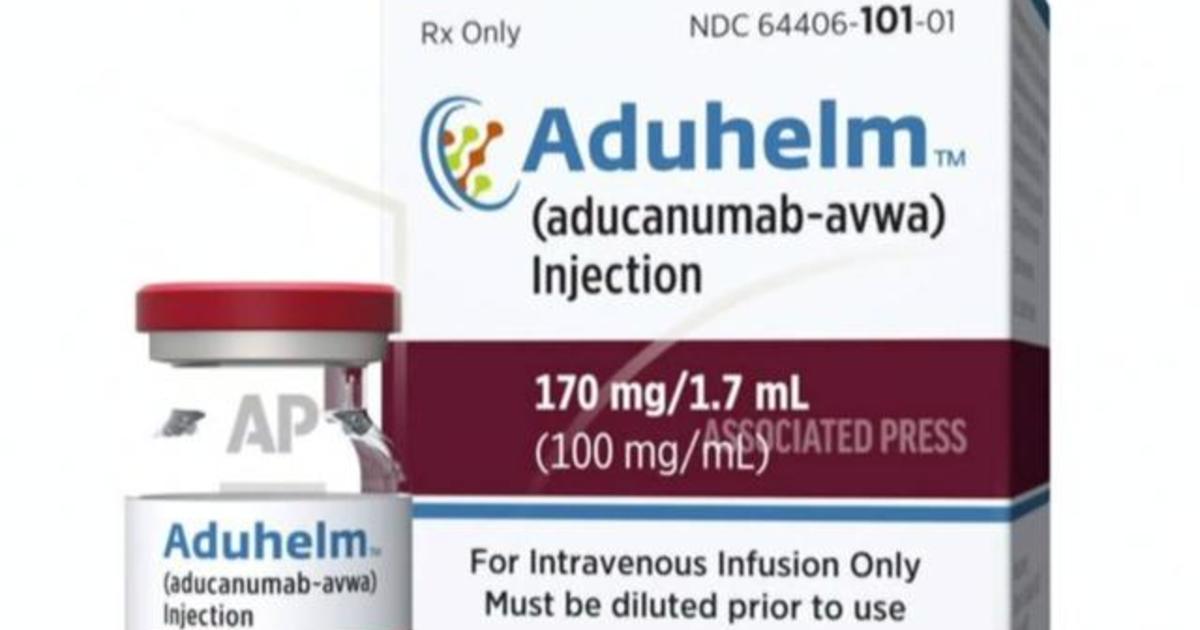
Janet Woodcock, the acting commissioner of U.S. Food and Drug Administration, said on Friday she is calling for an investigation into links between FDA staff and pharmaceutical company Biogen prior to the agency's approval of a controversial Alzheimer's drug.
"Given the ongoing interest and questions, today I requested that [the Office of Inspector General at the U.S. Department of Health & Human Services] conduct an independent review and assessment of interactions between representatives of Biogen and FDA during the process that led to the approval of Aduhelm," Woodcock wrote on Twitter. Aduhelm, the brand name of the Biogen drug, was approved a month ago by the FDA, making it the first new drug for Alzheimer's disease in nearly 20 years cleared for commercial use by government health officials.More Related News




