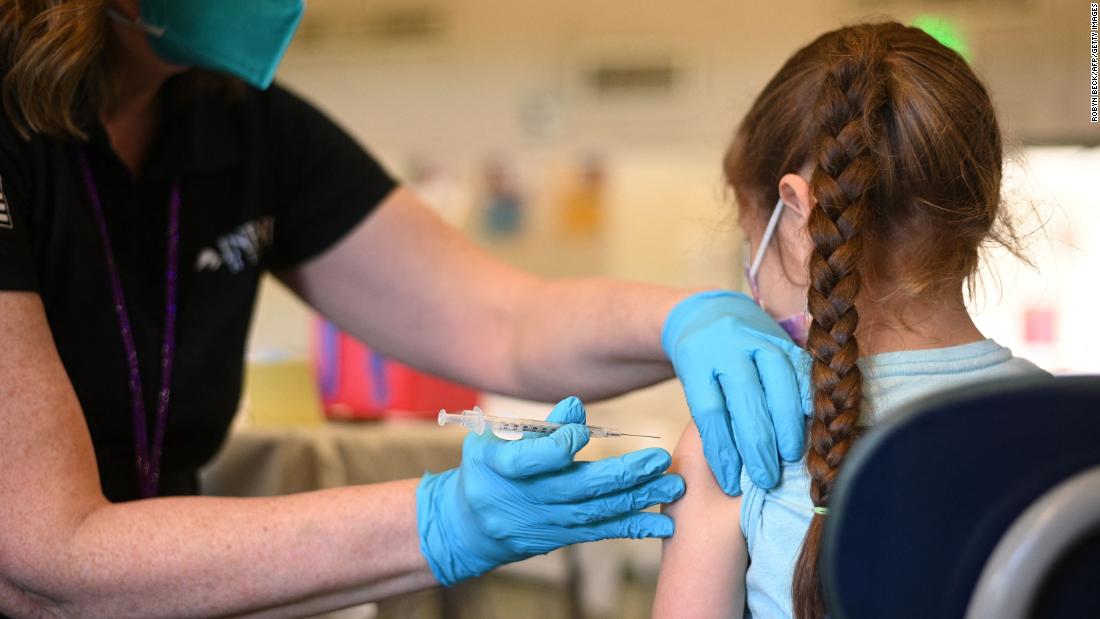
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11
CNN
The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36 times in this age group.
"While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer term effects, even following initially mild disease," FDA Commissioner Dr. Robert Califf said in a news release Tuesday. "The FDA is authorizing the use of a single booster dose of the Pfizer-BioNTech COVID-19 Vaccine for children 5 through 11 years of age to provide continued protection against COVID-19.

Texas judge orders Attorney General Ken Paxton’s divorce records unsealed amid heated Senate primary
Court documents detailing the divorce of Republican U.S. Senate candidate and Texas Attorney General Ken Paxton and his wife, state Sen. Angela Paxton, were released Friday by order of a judge, months after she filed citing “biblical grounds.”












