FDA approves new drug that may help stop and even reverse a rare, fatal condition that doctors call a ‘ticking time bomb’
CNN
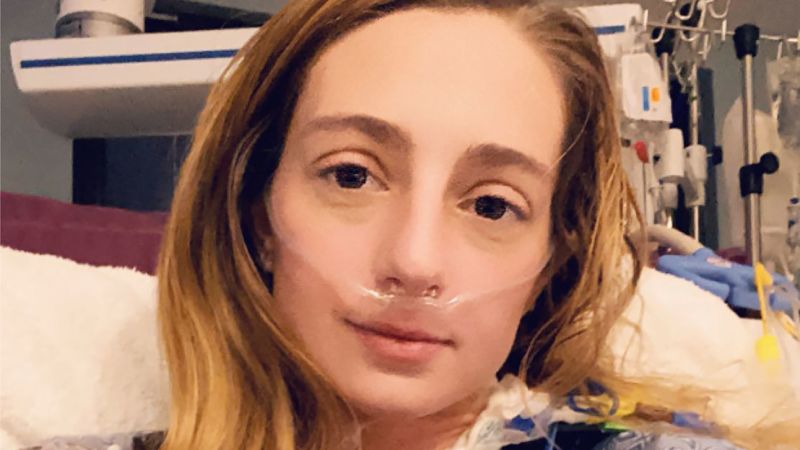
When doctors told Katrina Barry that she had a rare and serious condition called pulmonary arterial hypertension, or PAH, they warned her not to Google it.
When doctors told Katrina Barry that she had a rare and serious condition called pulmonary arterial hypertension or PAH, they warned her not to Google it. Come on, she thought; they wanted a young woman who was bound for graduate school, who had survived a transatlantic plane flight while having a heart attack and now open-heart surgery, not to look up the condition that kept trying to kill her? Waiting for her on the internet was some chilling information. PAH affects about 500 to 1,000 Americans each year, often women between the ages of 30 and 60, according to the American Lung Association. Barry, who was 25 at the time, learned from her reading that she had two to five years to live, based on how severe her condition already was. Then her medical team offered her a potential lifeline: a first-of-its-kind experimental drug called sotatercept that corrals a growth factor that is overproduced by people with PAH, potentially changing the underlying biology of the disease. She signed up for a study to test the medication, given every three weeks by injection under the skin.

 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game