MoreBack to News Headlines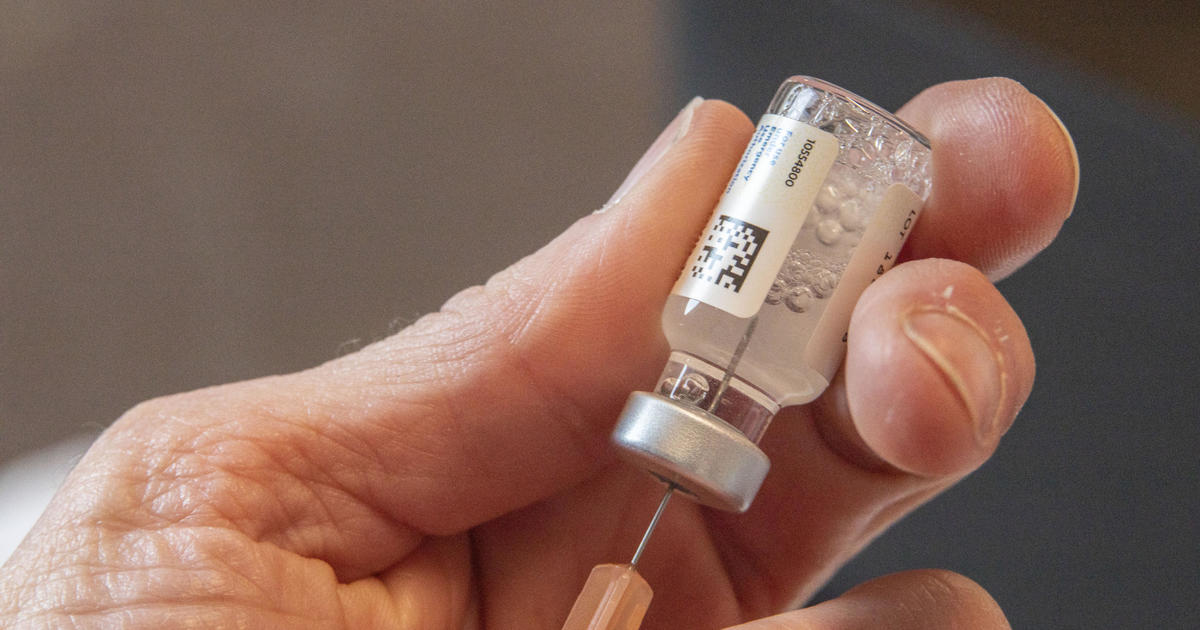

CDC recommends resuming use of Johnson & Johnson COVID-19 vaccine
CBSN
A panel of advisers to the Centers for Disease Control and Prevention voted Friday to recommend resuming use of Johnson & Johnson's Janssen vaccine to protect against COVID-19. The shots are expected to be accompanied by a new warning about an increased risk of rare but serious blood clots for adult women under 50.
A federal health official told the panel Friday that vaccinations could resume once the CDC director approves its recommendations and the Food and Drug Administration publishes an update to its emergency use authorization for the vaccine. A total of three women have died from the clotting disorder after receiving the Janssen vaccine, out of 16 cases like it in total among women in the U.S, the CDC told its panel of advisers on Friday. Seven remain hospitalized and five have been released. Most were under the age of 50.More Related News




















 Run 3 Space | Play Space Running Game
Run 3 Space | Play Space Running Game Traffic Jam 3D | Online Racing Game
Traffic Jam 3D | Online Racing Game Duck Hunt | Play Old Classic Game
Duck Hunt | Play Old Classic Game