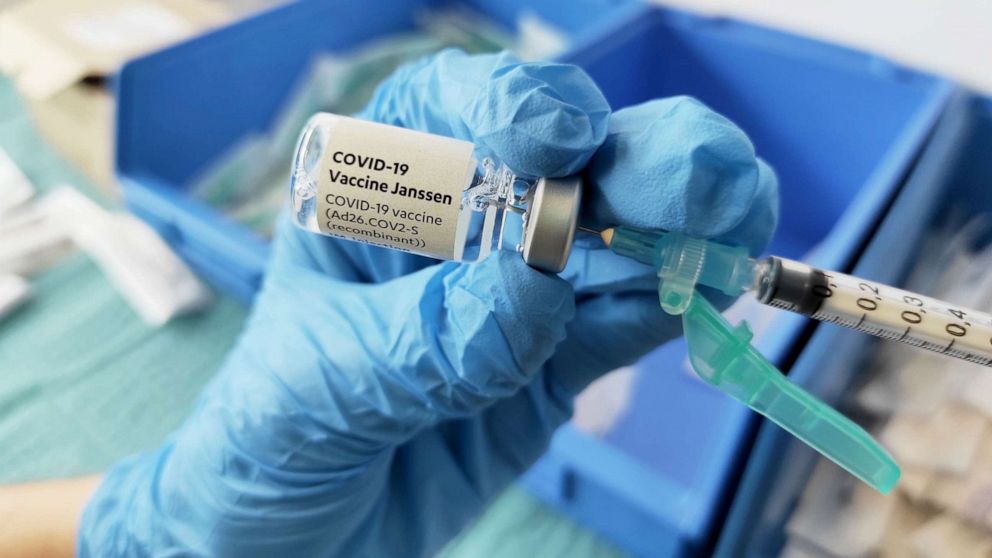
CDC, FDA lift pause on Johnson & Johnson vaccine with warning of risks
ABC News
Citing an urgent need to vaccinate the country quickly, a government advisory panel recommended the nation resume injections of the Johnson & Johnson vaccine.
The Centers for Disease Control and Prevention and Food and Drug Administration have lifted a 10-day pause on the Johnson & Johnson vaccine, but will issue a fact sheet to medical providers warning them of the potential for extremely rare but serious blood clots. There will be little delay in the J&J one-dose shots being offered again, officials said. Dr. Peter Marks, director of FDA's Center for Biologics Evaluation and Research, said shots should be able to resume "shortly after this announcement. ... I would expect them to resume probably by tomorrow morning, even." Marks said the FDA had been prepping revised fact sheets over the past few days in advance for both providers and patients.More Related News
