New COVID-19 breath test approved in U.S. Here’s how it could be useful
Global News
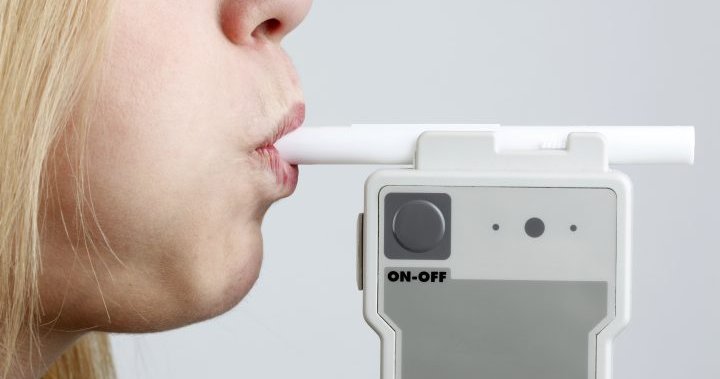
A non-invasive devise using breath samples can test for COVID-19 in three minutes, but experts say there is a trade-off to the convenience and speed.
A new COVID-19 screening tool promises to make testing easier, faster and painless, relying on the simple act of breathing.
The U.S. Food and Drug Administration (FDA) on Wednesday issued emergency use authorization for what it said is the first device that can detect COVID-19 in breath samples.
Health Canada told Global News on Friday it has not yet received an application for the test, but Canadian experts say this non-invasive tool could not only make testing easier but could reduce transmission rates in key settings.
“The biggest benefit of a breath test, if it’s not too expensive and it is sensitive enough, is that everybody breathes and you don’t have to learn a whole lot more to do it,” said Dr. Lisa Barrett, an infectious diseases physician and researcher at Dalhousie University.
“It’s a test that’s adding to an already pretty good set of tools. Turn-around time would be one of the big benefits for it,” she added.
The use of rapid antigen tests that can be purchased and performed at home have become increasingly common in Canada.
With a sensitivity of 91 per cent, the breath test is more accurate than the rapid antigen test, said Dr. Raywat Deonandan, an epidemiologist and associate professor at the University of Ottawa.
“That’s pretty good. It’s better than the rapid antigen tests, which hover around 60 per cent these days. But it’s not ideal,” he told Global News.











